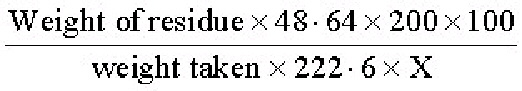S.I. No. 264/1957 -- Fertilisers, Feeding Stuffs and Mineral Mixtures Regulations, 1957.
1957
264
|
|
S.I. No. 264/1957:
FERTILISERS, FEEDING STUFFS AND MINERAL MIXTURES REGULATIONS, 1957.
|
|
|
FERTILISERS, FEEDING STUFFS AND MINERAL MIXTURES REGULATIONS, 1957.
|
|
|
I, PATRICK SMITH, Minister for Agriculture, in exercise of the powers conferred on me by section 11 of the Fertilisers, Feeding Stuffs and Mineral Mixtures Act, 1955 (No. 8 of 1955), hereby make the following regulations (the sanction of the Minister for Finance having been given with respect to the fee specified in Article 6 and the Minister for Industry and Commerce having been consulted with respect to Article 13) :
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Short title and commencement. |
|
|
1.--(1) These Regulations may be cited as the Fertilisers, Feeding Stuffs and Mineral Mixtures Regulations, 1957.
|
|
|
(2) These Regulations shall come into operation on the 1st day of January, 1958.
|
|
|
Definitions. |
|
|
2. In these Regulations--
|
|
|
" the Act " means the Fertilisers, Feeding Stuffs and Mineral Mixtures Act, 1955 (No. 8 of 1955) ;
|
|
|
" complex fertiliser " means any fertiliser which contains not less than two of the elements nitrogen, phosphorus and potassium and in which any such elements are associated either naturally or by chemical means only ;
|
|
|
" liming material " means any fertiliser manufactured for use as a corrective of acidity in the soil ;
|
|
|
" mixed fertiliser " means any fertiliser which has been manufactured by mixing together two or more substances and which contains not less than two of the elements nitrogen, phosphorus and potassium ;
|
|
|
" single fertiliser " means any fertiliser which contains one only of the elements nitrogen, phosphorus and potassium ;
|
|
|
" superphosphate " means any fertiliser which has been produced by the treatment of rock phosphate with phosphoric acid or with sulphuric acid or with both phosphoric acid and sulphuric acid.
|
|
|
Particulars to be contained in statement. |
|
|
3.--(1) A statement given for the purposes of subsection (1) of section 2 of the act in relation to any article other than a feeding stuff or a compound feeding stuff specified in the First Schedule to these Regulations shall contain the following particulars only :
|
|
|
(a) the business name and address of the seller of the article to which the statement relates,
|
|
|
(b) the date on which the article was sold to the purchaser,
|
|
|
(c) the name under which the article was sold,
|
|
|
(d) the identifying marks (if any) on the container or on a label affixed to the container of the article, and
|
|
|
(e) such particulars of the article as are indicated in the next paragraph.
|
|
|
(2) The particulars referred to in subparagraph (e) of the foregoing paragraph are as follows, except that in the case of the articles mentioned in subparagraphs (b), (c) and (d) of this paragraph particulars as formerly prescribed under Article 9 of the Emergency Powers (Manufacture and Sale of Fertilisers) Order, 1944 (No. 49 of 1944), shall continue to be given for the period ending 30th June, 1958 :
|
|
|
(a) if the article is a liming material--
|
|
|
(i) the minimum neutralising value of the article, and in the case of ground limestone--
|
|
|
(ii) the amount of the article that will pass through a 1/8 inch sieve conforming to Irish Standard 24 : 1950, and
|
|
|
(iii) the minimum amount of the article that will pass through a No. 100 sieve conforming to that Standard ;
|
|
|
(b) if the article is basic slag--
|
|
|
(i) the minimum amounts of--
|
|
|
citric acid soluble phosphorus, and citric acid
|
|
|
insoluble phosphorus,
|
|
|
contained in the article, and
|
|
|
(ii) the minimum amount of the article that will pass through a No. 100 sieve conforming to Irish Standard 24 : 1950 ;
|
|
|
(c) if the article is superphosphate or a mixture of which superphosphate is the only source of phosphorus--
|
|
|
(i) the minimum amounts (if any) of--
|
|
|
nitrogen,
|
|
|
water soluble phosphorus, and
|
|
|
potassium,
|
|
|
contained in the article, and
|
|
|
(ii) the amount (if any) of boron contained in the article ;
|
|
|
(d) if the article is any other fertiliser--
|
|
|
(i) the minimum amounts (if any) of--
|
|
|
nitrogen,
|
|
|
water soluble phosphorus,
|
|
|
citrate soluble phosphorus,
|
|
|
citrate insoluble phosphorus, and
|
|
|
potassium,
|
|
|
contained in the article,
|
|
|
(ii) the amount (if any) of boron contained in the article, and,
|
|
|
in the case of ground rock phosphate,
|
|
|
(iii) the minimum amount of the article that will pass through a No. 100 sieve conforming to Irish Standard 24 : 1950 ;
|
|
|
(e) if the article is a feeding stuff or a compound feeding stuff and is described in the first column of the Second Schedule to these Regulations, the particulars mentioned in relation thereto in the second column of that Schedule ;
|
|
|
(f) if the article is a feeding stuff or a compound feeding stuff and is not described in the first column of the Second Schedule to these Regulations--
|
|
|
(i) the amount (if any) of oil,
|
|
|
(ii) the minimum amount (if any) of crude protein, and
|
|
|
(iii) the maximum amount (if any) of fibre, contained in the article ;
|
|
|
(g) if the article is a mineral mixture, the minimum amounts (if any) of--
|
|
|
calcium,
|
|
|
phosphorus,
|
|
|
salt (sodium chloride),
|
|
|
magnesium,
|
|
|
manganese (acid soluble),
|
|
|
cobalt,
|
|
|
copper,
|
|
|
iodine and
|
|
|
iron,
|
|
|
contained in the article.
|
|
|
(3) For the purposes of the foregoing paragraph--
|
|
|
(a) each amount shall be stated as a definite percentage of the weight of the article except that, in the case of betacarotene, the amount shall be stated in terms of parts per million as a definite proportion of the weight of the article ;
|
|
|
(b) nitrogen, phosphorus and potassium shall be stated in terms of the elements N, P and K, respectively ;
|
|
|
(c) the extent to which the article is capable of passing through the prescribed sieve or sieves shall be expressed as the fineness of the article ;
|
|
|
(d) neutralising value shall be stated in terms of calcium carbonate ;
|
|
|
(e) the amount of crude protein means the amount of nitrogen, other than ammoniacal or nitric nitrogen, if present, multiplied by 6·25 ;
|
|
|
(f) the amount of true protein means the amount of nitrogen, other than ammoniacal or nitric nitrogen or non-protein organic nitrogen, if present, multiplied by 6·25.
|
|
|
Time of giving statement. |
|
|
4. A statement for the purposes of subsection (1) of section 2 of the act shall be given on or before delivery of the article to the purchaser or as soon as reasonably practicable thereafter.
|
|
|
Limits of error in statement. |
|
|
5. The limits of error for the purposes of section 2 of the act shall be as follows :
|
|
|
|
|
|
|
Article
|
Limits of Error
|
|
(1) A liming material
|
Neutralising value, one-thirtieth of the amount stated ; amount that will pass through a prescribed 1/8 inch sieve, one-fortieth of the amount stated ; amount that will pass through a prescribed No. 100 sieve, one-twentieth of the amount stated.
|
|
|
|
|
|
|
|
Article
|
Limits of Error
|
|
(2) A single fertiliser (other than a liming material), or a complex fertiliser.
|
Nitrogen, one-fortieth of the amount stated ; phosphorus, one-twentieth of each amount stated ; potassium, one-twentieth of the amount stated ; amount that will pass through a prescribed No. 100 sieve, one-twentieth of the amount stated ; boron, one-fifth of the amount stated.
|
|
(3) A mixed fertiliser
|
Nitrogen, 0·4 per cent. plus one-twentieth of the amount stated ; phosphorus, 0·4 per cent. plus one-twentieth of each amount stated ; potassium, 0·4 per cent. plus one-twentieth of the amount stated ; boron, one-fifth of the amount stated.
|
|
(4) A feeding stuff or compound feeding stuff or a mineral mixture.
|
Oil, 0·75 per cent. or one-tenth of the amount stated whichever is the greater ; crude protein and true protein--
|
|
(a) if the amount stated does not exceed 20 per cent. of the weight of the article, 1 per cent. of the weight of the article,
|
|
(b) if the amount stated exceeds 20 per cent. but does not exceed 40 per cent. of the weight of the article, 2 per cent. of the weight of the article, and
|
|
(c) if the amount stated exceeds 40 per cent. of the weight of the article, one-twentieth of the amount stated ;
|
|
fibre, one-tenth of the amount stated ; calcium and phosphorus--
|
|
(a) if the amount stated does not exceed 1 per cent. of the weight of the article, 0·2 per cent. of the weight of the article,
|
|
(b) if the amount stated exceeds 1 per cent. but does not exceed 5 per cent. of the weight of the article, 0.3 per cent. of the weight of the article, and
|
|
(c) if the amount stated exceeds 5 per cent. of the weight of the article, one-twentieth of the amount stated ;
|
|
salt (sodium chloride), one-twentieth of the amount stated ; magnesium, one-tenth of the amount stated ; manganese, cobalt, copper, iodine and iron, one-half of the amount stated ; betacarotene, one-fifth of the amount stated.
|
|
|
|
Application to have sample taken for analysis. |
|
|
6.--(1) The period within which an application under section 3 of the act may be made to the Minister shall be the period of twelve consecutive days commencing on the day on which the article sought to be sampled has been delivered to the purchaser or on which the relevant statement has been received by the purchaser, whichever is the later. For the purposes of this paragraph, an article consigned to a purchaser shall be deemed not to be delivered to him until it arrives at the place to which it is consigned, whether the consignment is by direction of the seller or the purchaser.
|
|
|
(2) An application under section 3 of the act shall be in writing and shall be accompanied by a remittance, payable to the Minister for the amount of the fee, which shall be ten shillings for each article sought to be sampled.
|
|
|
Manner of taking sample. |
|
|
7.--(1) The manner in which a sample shall be taken and dealt with where under the Act a sample is required to be taken in the prescribed manner shall be as specified in the subsequent paragraphs of this Article.
|
|
|
(2) Where the quantity of the article to be sampled does not exceed or appear to exceed 2 cwt. in weight or is all contained in one container, the sample may consist of such a portion of the quantity as is fairly representative of the whole and the sample in duplicate shall be not less than 280 millilitres in volume. A sample shall be considered representative when taken in accordance with the method set out in subparagraph (b) of paragraph (8) of this Article.
|
|
|
(3) In the case of articles in packages or other containers, unopened packages or containers shall be selected in preference to opened packages or containers for the purpose of the sample.
|
|
|
(4) A sample shall not be drawn from part of any quantity where that part bears the appearance of having received, in transit or after delivery, damage likely to have rendered it unrepresentative of the whole.
|
|
|
(5) It shall be assumed that the quantity to be sampled is composed of separate approximately equal parts and that the number of such parts is equivalent--
|
|
|
(a) in the case of a mineral mixture which is in the state of separate blocks, bricks or other pieces of uniform shape and similar size, to the number of blocks, bricks or pieces composing the quantity to be sampled,
|
|
|
(b) in the case of any other article which is in packages or other containers, to the number of packages or containers to be selected in accordance with subparagraph (a) of paragraph (8) of this Article, and
|
|
|
(c) in the case of an article in bulk, to the number of portions to be taken in accordance with paragraph (9) of this Article.
|
|
|
Packages, containers or portions for the purpose of the sample shall be selected one from each part and shall be drawn from different positions in each part.
|
|
|
(6) In every case the sampling shall be done as quickly as possible consistently with due care and the material shall not be exposed any longer than is necessary.
|
|
|
(7) If the article is a mineral mixture in the state of separate blocks, bricks or other uniform pieces, any one block, brick or uniform piece may be selected for the purpose of the sample. The selected block, brick or uniform piece shall be wrapped in a clean dry material and shall be finely broken on a clean dry smooth surface with a hammer or other suitable instrument. The fragments shall be thoroughly mixed together and the sample shall be taken in duplicate by transferring a quantity of the material approximately equal to 140 millilitres in volume into each of two clean dry bottles, each of which shall be provided with a close-fitting stopper or lid.
|
|
|
(8) If the article is not a mineral mixture in the state of separate blocks, bricks or other uniform pieces and is in packages or other containers--
|
|
|
(a) when the article is in packages or other containers and the quantity to be sampled exceeds or appears to exceed 2 cwt. in weight, a number of packages or containers shall be selected as follows :--
|
|
|
|
|
|
|
Quantity taken for sampling
|
But not fewer packages or containers than
|
|
Per cent.
|
|
|
Where the quantity exceeds one package or container and does not exceed 20 packages or containers
|
10
|
2
|
|
Where the quantity exceeds 20 packages or containers and does not exceed 60 packages or containers
|
5
|
2
|
|
Where the quantity exceeds 60 packages or containers and does not exceed 200 packages or containers
|
4
|
3
|
|
Where the quantity exceeds 200 packages or containers and does not exceed 500 packages or containers
|
3
|
8
|
|
Where the quantity exceeds 500 packages or containers
|
2
|
15
|
|
|
|
When the number of packages or containers to be selected according to the above percentage scale contains a fraction, that fraction shall be counted as a whole number ;
|
|
|
(b) if the article is not in a fluid or semi-fluid condition--
|
|
|
(i) where the quantity does not exceed 60 packages or containers, each selected package shall be emptied on a clean dry surface and flattened as near as possible to a uniform depth ; 10 portions shall then be taken from different parts of, and as near as possible to the full depth of, the material ;
|
|
|
(ii) where the quantity exceeds 60 packages or containers, one portion at least shall be taken from each selected package ;
|
|
|
(iii) in either case (whether or not the quantity exceeds 60 packages), the several portions so obtained shall then be thoroughly mixed together with a suitable instrument ; the composite sample so obtained shall be reduced to a suitable quantity not less than 280 millilitres in volume by the use of a mechanical divider or riffle ; alternatively, except in the case of granular fertilisers, the reduction of the composite sample may be effected by placing the sample on a pliable surface and successively folding and unfolding that surface, the folding to be reversed occasionally so as to discard part of the material ; the process to be continued until the material remaining on the pliable surface is reduced to the required quantity ; a sample shall then be taken in duplicate by transferring a quantity of the material approximately equal to 140 millilitres in volume into each of two clean dry bottles, each of which shall be provided with a close-fitting stopper
or lid ;
|
|
|
(iv) where any appreciable portion of the material appears to be mouldy or to have undergone decomposition such as would render it unsuitable for the purpose for which it purports to be intended, separate samples shall be drawn of the apparently unsuitable portion and of the residue of the material, respectively ;
|
|
|
(c) if the article is in a fluid or semi-fluid condition, the selected containers shall be well shaken or the contents agitated or otherwise treated to ensure uniformity ; an approximately equal proportion of the fluid shall then be taken immediately from each of the selected containers, emptied into a clean, dry non-metallic vessel and well mixed by stirring or shaking ; the sample shall be taken in duplicate by immediately pouring approximately 140 millilitres of the mixture into each of two clean dry bottles, each of which shall be provided with a close-fitting stopper or lid.
|
|
|
(9) If the article is not a mineral mixture in the state of separate blocks, bricks or other uniform pieces and is in bulk, a number of portions shall be taken in accordance with the following scale :
|
|
|
|
|
|
|
|
Portions
|
|
Where the quantity exceeds 2 cwt. and does not exceed 10 tons
|
10
|
|
Where the quantity exceeds 10 tons and does not exceed 20 tons
|
20
|
|
Where the quantity exceeds 20 tons and does not exceed 100 tons--for each additional 5 tons or part thereof in excess of 20 tons
|
1
|
|
Where the quantity exceeds 100 tons--for each additional 10 tons or part thereof in excess of 100 tons
|
1
|
|
|
|
The portions shall be treated and the sample drawn in the manner described in subparagraph (b) of paragraph (8) of this Article.
|
|
|
(10) Each of the two bottles containing the sample in duplicate shall be so secured and sealed that it cannot be opened without breaking the seal. The authorised officer taking the sample shall affix to the outside of each bottle an adhesive label initialled by him and marked with the name of the article, the date and place of the sampling and a distinguishing mark or number. The label may also be initialled by the person on whose premises the sample is taken, or his representative.
|
|
|
(11) One of the sealed sample bottles shall be transmitted by the authorised officer to the seller of the article from which the sample was taken.
|
|
|
Period within which sample may be taken. |
|
|
8. For the purposes of paragraph (a) of subsection (4) of section 3 of the act, the period after the expiration of which a sample may not be taken under section 3 of the act shall be the period of twenty-one consecutive days commencing on the day on which the article has been delivered to the purchaser or on which the relevant statement has been received by the purchaser, whichever is the later. For the purposes of this paragraph an article consigned to a purchaser shall not be deemed to be delivered to him until it arrives at the place to which it is consigned, whether the consignment is by direction of the seller or the purchaser.
|
|
|
Manner of analysis of fertilisers. |
|
|
9.--(1) The manner in which a sample of a fertiliser shall be analysed for the purposes of the Act shall be as specified in the subsequent paragraphs of this Article.
|
|
|
(2) Determination of moisture (loss on drying) shall be effected as follows : A representative portion of the sample shall be weighed, with precautions against loss of moisture. It shall then be dried in a ventilated oven maintained at a temperature between 100°C. and 105°C., until 2 successive weighings after cooling in a desiccator, at intervals of not less than 3 hours in the oven, show an increment of loss of not more than 0·2 per cent. of the original weight. The loss in weight, calculated as a percentage of the original weight, shall be taken to be the moisture content.
|
|
|
Determination of fineness.
|
|
|
Ground limestone.
|
|
|
(3) (a) In the case of ground limestone, determination of fineness shall be effected as follows : a quantity of the dried material, approximately 100 grams in weight, weighed to an accuracy of 0·1 gram, shall be sifted through a 1/8 inch test sieve conforming to Irish Standards 24 : 1950. Soft lumps shall be broken down with the fingers but shall not be rubbed on the sieve. Any residue remaining on the sieve shall be weighed to an accuracy of 0·1 gram, and the difference between its weight and the weight of the dried material taken for the test shall be expressed as a percentage of the latter weight and shall be reported as indicating the extent to which the material passed through the sieve.
|
|
|
The material which has passed through the 1/8 in. test sieve shall be transferred to a No. 100 test sieve conforming to Irish Standard 24 : 1950 and shall be sifted continuously for 10 minutes, with occasional tapping of the sides of the sieve. The material which has then passed through the No. 100 test sieve shall be weighed to an accuracy of 0·1 gram and the result shall be expressed as a percentage of the weight of the dried material taken for the test and shall be reported as indicating the percentage of the material which passed through the No. 100 test sieve.
|
|
|
Basic slag and ground rock phosphate.
|
|
|
(b) In the case of basic slag and ground rock phosphate, determination of fineness shall be effected as follows : the sample shall be mixed and an adequate quantity shall be dried at 100°C. and 20 grams thereof shall then be transferred to a No. 100 test sieve (fitted with a receiver) conforming to Irish Standard 24 : 1950. The sieve shall then be shaken for 10 minutes with occasional tapping of the sides of the sieve. At the end of 10 minutes, the material which has passed through into the receiver shall be carefully brushed out into a suitable vessel and weighed. The receiver shall be replaced and the shaking repeated for another 10 minutes, when the sifted matter shall again be removed, mixed with the first portion and weighed. The process shall be repeated until not more than 0·2 per cent. is sifted during 10 minutes.
|
|
|
Soft lumps which can be caused to crumble by application of the fibres of a bristle brush shall be broken down after each shaking period, but in such manner that the hard parts of the brush do not come into contact with the sieve. The brush shall not be used in any way to brush particles through the sieve.
|
|
|
Preparation of the sample for analysis (other than determination of moisture, fineness or moisture and fineness).
|
|
|
(4) (a) Preparation of the sample for analysis (other than determination of moisture, fineness or moisture and fineness) shall be effected as specified in the subsequent subparagraphs of this paragraph.
|
|
|
Powdered fertilisers in dry, or moderately dry, condition.
|
|
|
(b) In the case of powdered fertilisers in a dry, or moderately dry, condition, the sample shall be passed through a sieve having apertures about one millimetre square. Adventitious materials which cannot be conveniently crushed, e.g., fragments of metal in basic slag, shall be removed and allowed for.
|
|
|
Other substances dry enough to powder.
|
|
|
(c) Other substances which are dry enough to powder, but which are not in a fine condition, shall be pulverised until the sample passes through a sieve having apertures about one millimetre square.
|
|
|
Moist fertilisers.
|
|
|
(d) Moist fertilisers which do not admit of being passed through a sieve shall be thoroughly mixed by the most suitable means.
|
|
|
Substances gaining or losing water during pulverising or mixing.
|
|
|
(e) In the case of substances which gain or lose water during the process of pulverising or mixing, the proportion of water shall be determined in the coarse and in the powdered condition respectively, and the results of the analysis of the powdered sample shall be calculated to the water content of the original coarse substance.
|
|
|
Crystalline or saline materials.
|
|
|
(f) Crystalline or saline materials, such as sulphate of ammonia, nitrate of soda or salts of potassium, may be prepared by being well mixed and rapidly ground in a stoneware mortar, the portion finally reserved for analysis being specially finely ground.
|
|
|
Procedure after sieving and mixing or after mixing.
|
|
|
(g) When the sample has been passed through the sieve and thoroughly mixed, or, if not passed through the sieve, has been thoroughly mixed, a part of it not being less than 100 grams shall be placed in a stoppered bottle and from this the portions for analysis shall be weighed.
|
|
|
Determination of nitrogen.
|
|
|
(5) (a) Determination of nitrogen shall be effected as specified in the subsequent subparagraphs of this paragraph.
|
|
|
Presence or absence of nitrates.
|
|
|
(b) The presence or absence of nitrates shall first be ascertained.
|
|
|
Nitrogen (organic and ammoniacal) in absence of nitrates.
|
|
|
(c) Nitrogen (organic and ammoniacal) in absence of nitrates shall be determined as follows : a weighed portion of the sample shall be transferred to a Kjeldahl digestion flask, 25 millilitres of concentrated sulphuric acid (or more if necessary) shall be added and the flask gently heated until frothing ceases. 10 grams of potassium or sodium sulphate (anhydrous) shall then be added and the flask further heated until the colour of the clear liquid ceases to diminish. The digestion shall be continued for an hour thereafter to ensure complete oxidation of the organic matter. The operation may be accelerated by the addition of a suitable catalyst (e.g., copper sulphate or mercury or a mixture of mercuric oxide and selenium) to the liquid in the digestion flask.
|
|
|
The quantity of ammonia present in the liquid shall be determined by distillation into standard acid after liberation with alkali and, where mercury or mercuric oxide has been used, with the addition also of sodium or potassium sulphide solution or sodium thiosulphate.
|
|
|
Nitrogen (total, i.e., organic, ammoniacal and nitric) when nitrates are present.
|
|
|
(d) (i) Nitrogen (total, i.e., organic, ammoniacal and nitric) when nitrates are present shall be determined as specified in the subsequent clauses of this subparagraph.
|
|
|
(ii) The presence or absence of more than traces of chlorides shall first be ascertained.
|
|
|
(iii) When chlorides are not present in more than traces, a weighed portion of the sample shall be transferred to a Kjeldahl digestion flask, 30 millilitres of concentrated sulphuric acid, containing 1 gram of salicylic acid or 1 gram of phenol, shall be added and the flask shall be shaken so as to mix its contents without delay. The shaking shall be continued at intervals during 10 minutes, the flask being kept cool, and then 10 grams of potassium or sodium sulphate (anhydrous) shall be added, together with either 5 grams of crystalline sodium thiosulphate or 2 grams of zinc dust. The flask shall be heated until the colour of the clear liquid ceases to diminish and for an hour thereafter. A further quantity of concentrated sulphuric acid may be added if necessary. Copper sulphate or mercury or a mixture of mercuric oxide and selenium may be used as indicated in subparagraph (c) of this paragraph.
|
|
|
The quantity of ammonia shall be determined as described in subparagraph (c) of this paragraph.
|
|
|
(iv) When chlorides are present in more than traces, about 2 grams of the sample, accurately weighed, and 3 grams of finely powdered Devarda metal shall be transferred to a 500 millilitre Kjeldahl digestion flask, and the sides of the flask shall be washed down with 50 millilitres of water. The flask shall be closed with a rubber stopper provided with (I) a tap funnel and (II) a delivery tube connected with a U-tube (with bulbs) containing 10 millilitres of 10 per cent. v/v sulphuric acid. 5 millilitres of sodium hydroxide solution (specific gravity 1·40) shall be added through the tap funnel. The flask shall be allowed to stand for half an hour and then heated to just short of boiling point for a further hour. At the end of this digestion the flask shall be cooled and 20 millilitres of sulphuric acid (specific gravity 1·50) shall be added through the tap funnel in such a manner that the sides of the Kjeldahl flask shall be washed down by the acid. The rubber
stopper shall now be removed and the contents of the U-tube (with bulbs) shall be washed into the Kjeldahl flask. 25 millilitres of concentrated sulphuric acid shall be added to the flask and the flask shall be heated until all the water has boiled off. 10 grams of potassium or sodium sulphate (anhydrous) shall then be added and the flask further heated until the colour of the clear liquid ceases to diminish. The digestion shall be continued for 2 hours thereafter to ensure complete oxidation of the organic matter. Copper sulphate or mercury or a mixture of mercuric oxide and selenium may be used as indicated in subparagraph (c) of this paragraph.
|
|
|
The quantity of ammonia shall be determined as described in subparagraph (c) of this paragraph.
|
|
|
Nitrogen in form of ammonium salts.
|
|
|
(e) (i) Nitrogen in form of ammonium salts shall be determined as specified in the subsequent clauses of this paragraph.
|
|
|
(ii) In absence of organic matter, a weighed portion of the sample shall be dissolved in water and made up to a definite bulk. An aliquot part of the solution shall be transferred to a distillation flask and the quantity of ammonia shall be determined as described in subparagraph (c) of this paragraph.
|
|
|
(iii) In presence of organic matter, a weighed portion of the sample shall be well shaken with water, filtered, the insoluble matter thoroughly washed, the filtrate transferred to a distillation flask, diluted with water to about 200 millilitres, 5 grams of magnesium oxide (free from carbonates) added, and the quantity of ammonia determined as described in subparagraph (c) of this paragraph.
|
|
|
(iv) In the case of mixed or complex fertilisers containing calcium carbonate with small quantities of ammonium salts, the portion taken for analysis must be dissolved in or shaken with hydrochloric acid instead of water.
|
|
|
Nitrogen in nitrates.
|
|
|
(f) Nitrogen in nitrates shall be determined as follows : a weighed portion of the sample shall be dissolved in water and made up to a definite bulk. An aliquot part of the solution shall be transferred to a flask and a quantity of finely powdered Devarda metal (not less than six times the weight of the sample present in the aliquot part taken) added. An excess of concentrated alkali shall then be added and the flask at once connected with a distillation apparatus. After standing for 30 minutes to allow the reaction to proceed, heating gently if necessary, the ammonia shall be determined by distillation into standard acid.
|
|
|
Distillation shall proceed for at least one hour.
|
|
|
Control experiment in determination of nitrogen.
|
|
|
(g) The materials used in any of the methods described in this paragraph shall be examined as to their freedom from nitrogen by means of a control experiment carried out under similar conditions with the same quantities of the reagents which have been employed in the actual analysis, in the case of subparagraph (c) of this paragraph one gram of pure sugar being used in place of the weighed portion of the sample. The quantity of standard acid found to have been neutralised in the control experiments shall be deducted from the total quantity of acid neutralised in the distillation of the sample.
|
|
|
Determination of phosphorus.
|
|
|
(6) (a) Determination of phosphorus shall be effected as specified in the subsequent subparagraphs of this paragraph.
|
|
|
Water soluble phosphorus.
|
|
|
(b) (i) Water soluble phosphorus shall be determined as specified in the subsequent clauses of this subparagraph.
|
|
|
(ii) Reagents shall be prepared as follows :--
|
|
|
(a) Citric Molybdate reagent : 54 grams of molybdic anhydride shall be stirred with 200 millilitres of water and 11 grams of sodium hydroxide until the molybdic anhydride dissolves ; the mixture shall be heated to assist solution. 60 grams of citric acid shall be dissolved in a further 300 millilitres of water and 140 millilitres of concentrated hydrochloric acid added to it. The molybdate solution shall then be poured into the acid solution, which shall be stirred throughout the addition.
|
|
|
The whole shall be cooled and if necessary filtered through a pad of filter paper pulp. The filtrate shall be diluted with water to a litre and the slight green or blue colour discharged by the dropwise addition of a 1 per cent. solution of potassium bromate. This reagent shall be stored in the dark.
|
|
|
(b) Quinoline reagent : 60 millilitres of concentrated hydrochloric acid shall be diluted to 400 millilitres with water and heated to between 70° and 80°C., 50 millilitres of quinoline (laboratory reagent quality) shall be poured into the diluted acid in a thin stream with continuous stirring. After the quinoline has dissolved the solution shall be cooled, diluted to a litre with water, and filtered through a pad of filter paper pulp. The pulp shall not be washed.
|
|
|
(c) Indicator : 0·10 gram of thymol blue shall be dissolved in 2·2 millilitres of 0·1 N sodium hydroxide solution, 50 millilitres of methylated spirits shall be added and the whole diluted to 100 millilitres with water and mixed well. 3 volumes of this solution shall then be mixed with 1 volume of 0·1 per cent. solution of phenolphthalein in 60 per cent. ethyl alcohol.
|
|
|
(iii) The procedure shall be as follows : 20 grams of the sample shall be continuously agitated for 30 minutes in a litre flask with 800 millilitres of water at room temperature of about 20°C. The flask shall then be filled to the mark with water, shaken, and the contents filtered. The phosphorus shall be determined in the filtrate by the following method :
|
|
|
An aliquot part (chosen so as to contain less than 30 milligrams of phosphorus and preferably about 20 milligrams of phosphorus) of the solution obtained as above described shall be transferred to a conical flask of about 500 millilitres capacity fitted with a stopper or a rubber bung and marked at 150 millilitres. The aliquot part shall be diluted to 150 millilitres and 50 millilitres of reagent (a) shall be added. The solution shall be heated to incipient ebullition and kept at this temperature for 3 minutes, then heated to the boiling point. 25 millilitres of reagent (b) shall be slowly run in from a burette, with constant swirling throughout, the first few millilitres being added dropwise, and the rest in a slow stream keeping the solution gently boiling throughout. The flask shall then be allowed to stand in a bath of boiling water for 5 minutes, after which it shall be cooled in running water to 15°C. The contents of the flask shall be
filtered through a pad of filter paper pulp, and the flask, precipitate and filter shall be washed with successive small volumes of cold water until they are free from acid. The filter-pad and precipitate shall then be transferred to the original flask, using not more than 100 millilitres of water. A few drops of a dilute neutral solution of a surface active agent (e.g., a 0·5 per cent. solution of sodium dodecyl benzene sulphonate) may be added if desired to facilitate the dispersal of the precipitate. The flask shall then be stoppered and shaken vigorously until the pulp and precipitate are thoroughly dispersed. The stopper shall be removed and washed with distilled water, the washings being returned to the flask, then an accurately measured volume of 0·5 N sodium hydroxide solution (free from carbon dioxide) shall be added, the volume added being such that it contains an excess of sodium hydroxide beyond that necessary to dissolve the precipitate. The stopper or bung shall
be replaced, and the flask shaken until the precipitate is completely dissolved. The excess of sodium hydroxide shall be titrated with 0·5 N hydrochloric acid solution, about 0·5 to 1 millilitre of reagent (c) being added and the end point being taken as the sharp change from green-blue to yellow.
|
|
|
The volume of 0·5 N hydrochloric acid used shall be deducted from the volume of 0·5 N sodium hydroxide, previously added, in order to ascertain the volume of 0·5 N sodium hydroxide equivalent to the phosphorus.
|
|
|
1 millilitre 0·5 N sodium hydroxide solution is equivalent to 0·596 milligrams of phosphorus. A " blank " determination shall be carried out on all the reagents, omitting only the sample, and using 0·1 N solutions of acid and alkali for the titration. The " blank " shall be calculated in terms of 0·5 N sodium hydroxide and the result first obtained corrected accordingly.
|
|
|
Total phosphorus in all substances except basic slag.
|
|
|
(c) (i) Total phosphorus in all substances except basic slag shall be determined as specified in the subsequent clauses of this subparagraph.
|
|
|
(ii) In the absence of organic matter, 5 grams of the finely ground sample (if the phosphorus content does not exceed 6 per cent.) or 2·5 grams (if the phosphorus content exceeds 6 per cent.) shall be transferred to a 300 millilitre beaker and mixed with 10 millilitres of water. The beaker shall be covered by a watch glass and 10 millilitres of concentrated hydrochloric acid shall be added slowly and this shall be followed by 5 millilitres of concentrated nitric acid. The beaker and contents shall be heated to incipient ebullition on a hot plate and kept at this temperature for 10 minutes. The contents of the beaker shall be diluted with 100 millilitres of water and boiled for 10 minutes. The solution shall be cooled and transferred to a 500 millilitre volumetric flask and diluted to volume with water. The contents of the flask shall be thoroughly shaken and shall be filtered into a dry beaker through a dry paper. The first 20 millilitres of the filtrate
shall be rejected. The phosphorus shall be determined in an aliquot part by the method described in, clause (iv) of this subparagraph.
|
|
|
(iii) In the presence of organic matter, 5 grams of the finely ground sample (if the phosphorus content does not exceed 6 per cent.) or 2·5 grams (if the phosphorus content exceeds 6 per cent.) shall be transferred to a platinum dish, intimately mixed with 1 gram of calcium oxide, and thoroughly wetted with a little water. The mixture shall be dried and then heated to a temperature not exceeding 500°C. until the bulk of the organic matter is destroyed. The residue in the dish after cooling shall be transferred to a 300 millilitre beaker and shall be well mixed with 10 millilitres of water. The beaker shall be covered by a watch glass and 12 millilitres of concentrated hydrochloric acid shall be added slowly and this shall be followed by 5 millilitres of concentrated nitric acid. The beaker and contents shall be heated to incipient ebullition on a hot plate and kept at this temperature for 10 minutes. The contents of the beaker shall be diluted with 100
millilitres of water and shall be boiled for 10 minutes. The solution shall be filtered into a 500 millilitre flask, the insoluble matter washed with hot water, and the insoluble matter and filter paper then transferred to a dish and incinerated to a carbon-free ash. The ash shall be heated for 5 minutes with dilute hydrochloric acid (1 part of concentrated acid to 4 parts of water), then transferred to the filtrate in the 500 millilitre flask, well mixed, cooled, and the volume adjusted to the mark with water. The contents of the flask shall be thoroughly shaken and shall be filtered into a dry beaker through a dry paper. The first 20 millilitres of the filtrate shall be rejected. The phosphorus shall be determined in an aliquot part by the method described in clause (iv) of this subparagraph.
|
|
|
(iv) 50 millilitres or 25 millilitres (chosen to contain less than 30 milligrams of phosphorus and preferably about 20 milligrams of phosphorus) of the solution obtained as above described shall be transferred to a conical flask of about 500 millilitres capacity filled with a stopper or a rubber bung and marked at 150 millilitres. The solution shall be diluted to about 100 millilitres and, if the sample does not contain calcium, 100 to 200 milligrams of pure calcium carbonate shall be added, then sodium hydroxide solution until a faint permanent precipitate or turbidity is formed. Dilute hydrochloric acid shall be added dropwise until the precipitate just dissolves. The solution shall then be diluted to 150 millilitres with water and the phosphorus determined as described in clause (iii) of subparagraph (b) of this paragraph beginning at the words " 50 millilitres of reagent (a) shall be added."
|
|
|
Water insoluble phosphorus.
|
|
|
(d) Water insoluble phosphorus shall be determined as follows : the quantity of phosphorus soluble in water as determined in accordance with subparagraph (b) of this paragraph shall be deducted from the quantity of phosphorus as determined in accordance with subparagraph (c) of this paragraph and the difference, if any, shall be taken as the quantity of insoluble phosphorus.
|
|
|
Total phosphorus in basic slag.
|
|
|
(e) (i) Total phosphorus in basic slag shall be determined as specified in the subsequent clauses of this subparagraph.
|
|
|
(ii) Reagents shall be prepared as follows :
|
|
|
(a) Citric molybdate reagent : 54 grams of molybdic anhydride shall be stirred with 200 millilitres of water and 11 grams of sodium hydroxide until the molybdic anhydride dissolves ; the mixture shall be heated to assist solution. 120 grams of citric acid shall be dissolved in a further 300 millilitres of water and 140 millilitres of concentrated hydrochloric acid added to it. The molybdate solution shall then be poured into the acid solution, which shall be stirred throughout the addition. The whole shall be cooled and if necessary filtered through a pad of filter paper pulp. The filtrate shall be diluted with water to a litre and the slight green or blue colour discharged by the dropwise addition of a 1 per cent. solution of potassium bromate. This reagent shall be stored in the dark.
|
|
|
(b) Quinoline reagent : 60 millilitres of concentrated hydrochloric acid shall be diluted to 400 millilitres with water and heated to between 70° and 80°C. 50 millilitres of quinoline (laboratory reagent quality) shall be poured into the diluted acid in a thin stream with continuous stirring. After the quinoline has dissolved the solution shall be cooled, diluted to a litre with water, and filtered through a pad of filter paper pulp. The pulp shall not be washed.
|
|
|
(c) Indicator : 0·10 gram of thymol blue shall be dissolved in 2·2 millilitres of 0·1 N sodium hydroxide solution, 50 millilitres of methylated spirits shall be added and the whole diluted to 100 millilitres with water and mixed well. 3 volumes of this solution shall then be mixed with 1 volume of 0·1 per cent. solution of phenolphthalein in 60 per cent. ethyl alcohol.
|
|
|
(iii) The procedure shall be as follows : 2·5 grams of the finely powdered sample shall be transferred to a 300 millilitre beaker, thoroughly mixed with 30 millilitres of water after which a further 70 millilitres of water shall be added with continuous stirring. The mixture shall be heated and, during stirring, 10 millilitres of concentrated hydrochloric acid shall be added dropwise followed by 5 millilitres of concentrated nitric acid. The whole shall be gently boiled for 10 minutes, cooled, transferred to a 250 millilitre volumetric flask, diluted to the mark with water and well mixed. This solution shall be filtered through a dry paper into a dry beaker, the first 20 or 30 millilitres of filtrate being rejected.
|
|
|
According to the expected phosphorus content 50 millilitres or 25 millilitres shall be transferred to a conical flask of about 500 millilitres capacity fitted with a stopper or a rubber bung and marked at 150 millilitres. The solution shall be diluted to about 100 millilitres with water heated almost to boiling and a 20 per cent. solution of sodium hydroxide added drop by drop until there is a faint permanent precipitate. A few drops of dilute hydrochloric acid shall be added to the now boiling solution to redissolve the precipitate.
|
|
|
The solution shall be diluted with water to 150 millilitres, 1 gram of citric acid added and then 50 millilitres of reagent (a). The solution shall be gently boiled for 3 minutes and then 25 millilitres of reagent (b) shall be slowly added from a burette, the flask being swirled continuously during the addition. The solution shall again be heated to boiling, and boiled gently for 2 minutes, after which it shall be kept hot but not boiling for a further 5 minutes, then cooled to 15°C.
|
|
|
The contents of the flask shall be filtered through a pad of filter paper pulp, and the flask, precipitate and filter shall be washed with successive small volumes of cold water until they are free from acid. The filter pad and precipitate shall be transferred to the original flask, not more than 100 millilitres of water being used in effecting the transfer. A few drops of a dilute neutral solution of a surface active agent (e.g., a 0·5 per cent. solution of sodium dodecyl benzene sulphonate) may be added if desired to facilitate the dispersal of the precipitate. The flask shall be stoppered and well shaken until the pulp and precipitate are thoroughly dispersed. The stopper shall be removed and washed with distilled water, the washings being returned to the flask, then an accurately measured volume of 0·5 N sodium hydroxide solution (free from carbon dioxide) shall be added, the volume added being such that it contains an excess of sodium hydroxide
beyond that necessary to dissolve the precipitate. The flask shall be shaken until the precipitate is completely dissolved, and the excess of sodium hydroxide titrated with 0·5 N hydrochloric acid solution, about 0·5 to 1 millilitre of reagent (c) being added and the end point being taken as the sharp change from green-blue to yellow.
|
|
|
The volume of 0·5 N hydrochloric acid used shall be deducted from the volume of 0·5 N sodium hydroxide previously added in order to ascertain the volume of 0·5 N sodium hydroxide equivalent to the phosphorus.
|
|
|
1 millilitre 0·5 N sodium hydroxide solution is equivalent to 0·596 milligrams of phosphorus. A " blank " determination shall be carried out on all the reagents, omitting only the sample, and using 0·1 N solutions of acid and alkali for the titration. The " blank " shall be calculated in terms of 0·5 N sodium hydroxide, and the result first obtained corrected accordingly.
|
|
|
Citric acid soluble phosphorus
|
|
|
(f) Citric acid soluble phosphorus shall be determined as follows : 5 grams of the sample shall be transferred to a stoppered bottle of about 1 litre capacity. 10 grams of citric acid shall be dissolved in water. The volume shall be made up to 500 millilitres at a temperature of about 20°C. and about 50 millilitres of this solution shall be added to the weighed portion of the sample in the bottle and the sample dispersed by vigorous stirring for a few minutes. The remainder of the citric acid solution shall be added and the bottle fitted into a mechanical shaking apparatus and continuously agitated during 30 minutes at a temperature of about 20°C. The solution shall then be filtered through a large rapid filter, the whole of the liquid being poured on the paper at once. If not clear the filtrate shall be again poured through the same paper.
|
|
|
The determination shall be carried out on 50 millilitres or 25 millilitres (according to the amount of phosphorus present) as described in clause (iii) of subparagraph (e) of this paragraph beginning at the words " According to the expected phosphorus " except that no citric acid shall be added before 50 millilitres of the citric molybdate solution are added.
|
|
|
Citric acid insoluble phosphorus.
|
|
|
(g) Citric acid insoluble phosphorus shall be determined as follows : he quantity of phosphorus soluble in citric acid, as determined in accordance with subparagraph (f) of this paragraph, shall be deducted from the quantity of phosphorus determined in accordance with subparagraph (e) of this paragraph, and the difference (if any) shall be taken as the quantity of phosphorus insoluble in citric acid.
|
|
|
Citrate soluble phosphorus.
|
|
|
(h) (i) Citrate soluble phosphorus shall be determined as specified in the subsequent clauses of this subparagraph.
|
|
|
(ii) Reagents shall be prepared as follows :--
|
|
|
(1) Ammonium citrate solution : 173 grams of citric acid shall be dissolved in 350 millilitres of distilled water in a 1-litre volumetric flask. 537 millilitres of ammonium hydroxide (specific gravity 0·960) shall be added slowly, the flask being cooled. When the flask and contents are cooled to 20°C. distilled water shall be added to the mark. The specific gravity of this solution shall be 1·082 to 1·083 and 1 millilitre of the solution shall contain 42 milligrams of nitrogen.
|
|
|
(2) Sulpho-molybdate solution : 100 grams of of ammonium sulphate shall be transferred to a 2-litre volumetric flask and 900 millilitres of concentrated nitric acid shall be added. The mixture shall be shaken by hand. (A complete solution of the ammonium sulphate is not necessary). 300 grams of ammonium molybdate shall be dissolved in 800 millilitres of boiling water. The solution shall be cooled to room temperature and added cautiously to the ammonium sulphate-nitric acid mixture, shaking well after each addition. The mixture shall be again cooled to room temperature and made up to the mark with water. After 2 or 3 days the solution shall be filtered and stored in a cool and dark place.
|
|
|
(3) Acid mixture : 750 millilitres of concentrated nitric acid shall be mixed with 800 millilitres of water ; 60 millilitres of concentrated sulphuric acid shall then be added.
|
|
|
(4) Nitric acid (specific gravity 1·19 to 1·21) : 500 millilitres of concentrated nitric acid shall be added to 700 millilitres of water.
|
|
|
(5) Ammonium nitrate solution : 20 grams of ammonium nitrate shall be dissolved in a litre of water and nitric acid shall be added until the solution is distinctly acid to litmus.
|
|
|
(iii) The procedure shall be as follows : 2 grams of the sample shall be transferred to a 500 millilitre Wagner shaking bottle. 100 millilitres of distilled water shall be added, followed by 100 millilitres of reagent (1). The bottle shall then be placed in an end over end shaking apparatus and rotated for 1 hour at 40 revolutions per minute. The solution shall then be made up to the mark with reagent (1). The solution shall then be filtered, the first 20 millilitres of the filtrate being discarded.
|
|
|
10 to 20 millilitres of the filtrate (containing not more than 10 milligrams of phosphorus) shall be transferred to a 250 to 300 millilitre beaker, water being added when necessary to give a final volume of 20 millilitres. 50 millilitres of reagent (3) or reagent (4) if the sample contains sufficient sulphate) shall then be added. The beaker shall be heated until the solution begins to boil. It shall then be removed from the heat and the solution stirred. 50 millilitres of reagent (2) shall immediately be added by means of a pipette taking care that the reagent does not touch the side of the beaker. Within 4 minutes after the addition of the reagent the solution shall be stirred for 30 seconds. The solution shall be allowed to stand overnight and filtered, with suction, through a weighed Gooch crucible. The beaker and crucible shall be washed with 4 x 10 millilitre portions of reagent (5). The Gooch crucible shall then be placed on a second (dry) suction
flask and washed first with 10 millilitres and then with 5 millilitres of acetone (analar quality). After the last washing, the suction shall be increased to remove the last traces of acetone from the precipitate and maintained for 1 minute more. The Gooch crucible shall then be removed and placed in a desiccator containing 40 per cent. sulphuric acid for 4 hours. The crucible shall be weighed and replaced in the desiccator until constant weight is obtained. The weight of the precipitate multiplied by 0·01426 shall be taken as the weight of phosphorus. From the result so obtained shall be deducted the quantity (if any) of water soluble phosphorus as determined as described in subparagraph (b) of this paragraph and the difference (if any) shall be taken as the quantity of citrate soluble phosphorus.
|
|
|
Citrate insoluble phosphorus.
|
|
|
(i) Citrate insoluble phosphorus shall be determined as follows : the quantity of phosphorus soluble in ammonium citrate solution, determined as described in subparagraph (h) of this paragraph ending with the words " The weight of the precipitate multiplied by 0·01426 shall be taken as the weight of phosphorus " shall be deducted from the quantity of phosphorus determined in accordance with subparagraph (c) of this paragraph and the difference (if any) shall be taken to be the quantity of phosphorus insoluble in ammonium citrate solution.
|
|
|
Determination of potassium
|
|
|
(7) (a) Determination of potassium shall be effected as specified in the subsequent subparagraphs of this paragraph.
|
|
|
Salts of potassium.
|
|
|
(b) Salts of potassium shall be determined as follows : a quantity of the sample, about 2·5 grams in weight, accurately weighed, shall be heated nearly to boiling point with 10 millilitres of concentrated hydrochloric acid and 50 millilitres of water, breaking down with a stirring rod any crystals or lumps. It shall be diluted with water to about 100 millilitres and shall be boiled gently for a few minutes. It shall then be cooled, made up to 250 millilitres, or to such larger volume that 50 millilitres shall contain about 0·025 to 0·08 gram of potassium, and filtered. The potassium shall be determined in the filtrate as described in subparagraph (e) of this paragraph.
|
|
|
Potassium in mixed fertilisers containing little or no organic matter.
|
|
|
(c) Potassium in mixed fertilisers containing little or no organic matter shall be determined as follows : a quantity of the sample, about 2·5 grams in weight, accurately weighed, shall be boiled for 30 minutes with 125 millilitres of water and 50 millilitres of saturated ammonium oxalate solution. If necessary, a small quantity of a potassium-free anti-foaming agent may be added. It shall then be cooled, a slight excess of ammonium hydroxide added, made up to 250 millilitres, or to such larger volume that 50 millilitres shall contain about 0·025 to 0·08 gram of potassium, and filtered. The potassium shall be determined in the filtrate as described in subparagraph (e) of this paragraph.
|
|
|
Potassium in mixed fertilisers containing organic matter.
|
|
|
(d) Potassium in mixed fertilisers containing organic matter shall be determined as follows : 10 grams of the sample shall be gently incinerated at a temperature not exceeding 500°C. in order to destroy organic matter. The residue shall be boiled for 30 minutes with 125 millilitres of water and 50 millilitres of saturated ammonium oxalate solution. It shall then be cooled, a slight excess of ammonium hydroxide added, made up to 500 millilitres, or to such larger volume that 50 millilitres shall contain about 0·025 to 0·08 gram of potassium, and filtered. The potassium shall be determined in the filtrate as described in subparagraph (e) of this paragraph.
|
|
|
Precipitation of potassium as potassium chloroplatinate.
|
|
|
(e) Precipitation of potassium as potassium chloroplatinate shall be effected as follows : 50 millilitres of the filtrate, or such smaller quantity diluted to 50 millilitres as shall contain about 0·025 to 0·08 gram of potassium shall be transferred to a digestion flask of capacity about 300 to 500 millilitres, together with 10 millilitres of concentrated nitric acid. If desired a small silica bead or granule weighing about 0·25 gram may be added to prevent bumping. This shall have been previously tared with a prepared Gooch crucible or sintered glass crucible having an average pore diameter of 5 to 15 microns. The mixture shall be boiled for 2 minutes and 10 millilitres of concentrated hydrochloric acid shall be added. It shall be boiled down to approximately 25 millilitres and 5 millilitres of concentrated hydrochloric acid and an excess of platinum chloride solution over that required by the total alkalies present shall be added. The platinum
chloride solution shall contain 0·5 gram of platinum in 10 millilitres. The mixture shall be boiled down to 10 to 15 millilitres, rotating the flask occasionally and 5 millilitres of concentrated hydrochloric acid added. The heat shall be reduced and the mixture boiled down to 3 to 5 millilitres (depending on the amount of precipitate), rotating the flask frequently near the end of the evaporation. The flask shall be removed from the heat and swirled to dissolve any soluble residue on the walls. It shall be cooled and 25 millilitres of 95 per cent. alcohol shall be immediately added so that it washes completely the neck of the flask. The flask shall be chilled under running water, swirled and allowed to stand for at least 5 minutes. The contents of the flask shall be decanted into the tared crucible, suction applied, and the precipitate, together with the silica bead or granule if used, shall be transferred to the tared crucible with a stream of 95 per cent. alcohol and thoroughly
washed. The suction shall be reduced and the precipitate carefully washed with 2 or 3 portions of 10 millilitres each of a 20 per cent. aqueous solution of ammonium chloride saturated with potassium chloroplatinate at the temperature of washing and filtered immediately before use. Suction shall be increased and the precipitate washed 4 to 5 times with 10 millilitre portions of the wash solution. Finally the precipitate shall again be washed thoroughly with 95 per cent. alcohol. The crucible and contents shall be dried at 100°C. and weighed. The precipitate shall be calculated to its equivalent of potassium by multiplying its weight by 0·16084.
|
|
|
Determination of boron.
|
|
|
(8) Determination of boron shall be effected as follows : a weighed quantity of the sample, from 2 to 5 grams depending on its probable boron content, shall be transferred to a 200 millilitre round-bottomed flask. 5 millilitres of orthophosphoric acid and 20 millilitres of methanol shall be added, and the flask shall be connected with a Liebig condenser by means of a short glass tube passing through the stopper of the flask. 100 millilitres of 95 per cent. methanol shall be placed in a second 200 millilitre round-bottomed flask, which shall then be set in a water bath and connected to the first flask by means of a short bent glass tube passing through the stopper of the second flask and connected by rubber tubing to a long bent glass tube passing through the stopper of the first flask and reaching almost to the bottom. A receiving flask shall be placed in position, and sufficient heat applied to the water bath to keep a steady flow of bubbles of methanol
passing through the first flask. In order to keep the volume in the first flask at about 25 millilitres, a little heat shall be applied. The distillation shall be continued until 100 millilitres of distillate is obtained (about 30 minutes). 2 or 3 drops of phenolphthalein and sufficient 0·1 N sodium hydroxide to produce a permanent pink colour shall then be added to the distillate. The receiving flask shall be stoppered, vigorously shaken and connected at once with the condenser by means of a splash head. The methanol shall be distilled off, using a water bath, until the volume is reduced to about 10 millilitres. The residue shall be transferred to a platinum or porcelain dish, using as little water as possible, and shall be evaporated to dryness on the water bath. The contents of the dish shall be ignited below redness (450°C.) and shall then be cooled and acidified with a few drops of N hydrochloric acid. 20 to 25 millilitres of distilled water shall then be added. The
solution shall be heated for 1 to 2 minutes on a steam bath and shall then be filtered into a 200 millilitre round-bottomed flask, the filter paper being thoroughly washed. The filtrate shall be diluted to 50 to 75 millilitres with distilled water. The flask shall be attached to an air condenser, and the contents shall be boiled gently for a few minutes to remove carbon dioxide. 3 or 4 drops of methyl red indicator shall then be added, followed by 0·1 N sodium hydroxide until the red colour just disappears. There shall then be added about 1 gram of mannitol, or a smaller quantity if only a little boron is thought to be present. 2 or 3 drops of phenolphthalein indicator shall be added, and the solution shall be titrated with 0·1 N sodium hydroxide. The reagents used for the analysis shall be tested by a " blank " determination. If the sodium hydroxide is free from carbon dioxide, the " blank " shall not be more than 0·2 millilitre. The result shall be calculated in
accordance with the formula, 1 millilitre of 0·1 N NaoH=0·00108 gram B., and shall be expressed in terms of percentage of boron.
|
|
|
Determination of the neutralising value of a liming material
|
|
|
(9) Determination of the neutralising value of a liming material shall be effected as follows : a representative portion of the sample shall be dried in oven maintained at a temperature between 100°C. and 105°C. until two successive weighings after cooling in a desiccator, at intervals of not less than 3 hours in the oven, show an increment of loss of not more than 0·1 per cent. of the original weight. The material shall then be ground (if necessary) to a degree of fineness which permits the whole amount to pass through a No. 60 test sieve conforming to Irish Standard 24 : 1950 and shall be placed in an airtight container. A quantity of the material about 1 gram in weight, weighed to an accuracy of 1 milligram, shall be transferred to an Erlenmeyer flask of about 250 millilitre capacity, and 50 millilitres of 0·5 N hydrochloric acid shall be added. A small funnel shall be placed in the neck of the flask. The contents shall be heated, maintained at
gentle boiling for 5 minutes, and then cooled. The residual acid shall be determined by titration against 0·25 N sodium hydroxide, using phenolphthalein as indicator. The weight of calcium carbonate equivalent to the weight of hydrochloric acid which has been neutralised by the material shall be calculated and shall be expressed as a percentage of the weight of the material. This result shall be reported as the neutralising value.
|
|
|
Manner of analysis of feeding stuffs, compound feeding stuffs and mineral mixtures. |
|
|
10.--(1) The manner in which a sample of a feeding stuff, compound feeding stuff or mineral mixture shall be analysed for the purposes of the Act shall be as specified in the subsequent paragraphs of this Article.
|
|
|
Determination of moisture (loss on drying).
|
|
|
(2) Determination of moisture (loss on drying) shall be effected as follows : a representative portion of the sample shall be weighed, with precautions against loss of moisture. It shall then be dried in a ventilated oven maintained at a temperature between 100°C. and 105°C. until two successive weighings after cooling in a desiccator, at intervals of not less than 3 hours in the oven, show an increment of loss of not more than 0·2 per cent. of the original weight. The loss in weight, calculated as a percentage of the original weight, shall be taken to be the moisture content.
|
|
|
(3)
|
|
|
Preparation of the sample for analysis (other than determination of moisture).
|
|
|
(a) Preparation of the sample for analysis (other than determination of moisture) shall be effected as specified in the subsequent subparagraphs of this paragraph.
|
|
|
Sample in fine condition.
|
|
|
(b) If the sample is in a fine condition and passes through a sieve having apertures about one millimetre square, it shall be thoroughly mixed and a portion not less than 100 grams in weight shall be placed in a stoppered bottle. From this portion the quantities for analysis shall be taken.
|
|
|
Sample passing through sieve having apertures from two to three millimetres square.
|
|
|
(c) If the sample does not wholly pass through a sieve having apertures about one millimetre square and wholly passes through a sieve having apertures from two to three millimetres square, it shall be thoroughly mixed and a portion for the determination of the moisture shall be at once taken.
|
|
|
Sample in coarse condition.
|
|
|
(d) If the sample is in a coarse condition, it shall be carefully pulverised until the whole passes through a sieve having apertures from two to three millimetres square. It shall then be thoroughly mixed and a portion for the determination of the moisture shall be at once taken.
|
|
|
Further powdering.
|
|
|
(e) From the mixed sample as specified in subparagraph (c) of this paragraph, or from the coarsely crushed sample as specified in subparagraph (d) of this paragraph, a portion not less than 100 grams in weight shall be taken and further powdered and passed through a sieve having apertures about one millimetre square. The portion of the sample so prepared shall be placed in a stoppered bottle and from it the quantities for analysis shall be taken.
|
|
|
Original sample appreciably moist.
|
|
|
(f) If the original sample is appreciably moist, or if for any reason the operations of pulverisation and mixing are likely to result in loss or gain of moisture, the moisture in the bottled portion shall be determined as well as in the portion taken for that purpose under subparagraph (c) or (d) of this paragraph in order that the results of the analysis may be corrected to correspond with the sample in its original condition as regards moisture.
|
|
|
Mixing in other cases.
|
|
|
(g) Materials which cannot be conveniently pulverised or passed through a sieve shall be thoroughly mixed by the most suitable means.
|
|
|
Determination of oil.
|
|
|
(4) Determination of oil shall be effected as follows : a weighed quantity of the sample shall be placed in an extraction thimble, which shall then be placed in an extraction apparatus and extracted with petroleum ether (b. pt. 40-60°C.). At the end of 3 to 4 hours the thimble shall be removed from the apparatus, dried and its contents finely ground, preferably with sand, in a small mortar previously rinsed with petroleum ether. The substance shall then be returned to the thimble, the mortar being washed out with petroleum ether, and the extraction continued for another hour. The extract should be free from suspended matter. After evaporation of the solvent, the oil shall be dried at 100°C. and weighed.
|
|
|
Determination of protein.
|
|
|
(5)(a) Determination of protein shall be effected as specified in the subsequent subparagraphs of this paragraph.
|
|
|
Crude protein.
|
|
|
(b) (i) The percentage of crude protein shall be ascertained by multiplying the percentage of nitrogen, other than nitrogen present as ammoniacal or nitric nitrogen, by 6·25. The presence of nitrogen in these latter forms shall be tested for (according to the methods for determination of ammoniacal nitrogen and nitric nitrogen specified in subparagraph (5) of Article 9 of these Regulations) and the quantity so present, if any, shall be determined and deducted from the total nitrogen.
|
|
|
(ii) The determination of total nitrogen in the absence of nitrates shall be effected as follows :--
|
|
|
A weighed portion of the sample shall be transferred to a Kjeldahl digestion flask, 25 millilitres of concentrated sulphuric acid (or more if necessary) shall be added and the flask gently heated until frothing ceases. 10 grams of potassium or sodium sulphate (anhydrous) shall then be added and the flask further heated until the colour of the clear liquid ceases to diminish. The heating shall be continued for an hour thereafter to ensure complete oxidation of the organic matter. The operation may be accelerated by the addition of a suitable catalyst (e.g. copper sulphate or mercury or a mixture of mercuric oxide and selenium) to the liquid in the digestion flask.
|
|
|
The quantity of ammonia present in the liquid shall be determined by distillation into standard acid after liberation with alkali and, where mercury or mercuric oxide has been used, with the addition also of sodium or potassium sulphide solution or sodium thiosulphate.
|
|
|
The materials used shall be examined as to their freedom from nitrogen by means of a control experiment carried out under similar conditions with the same quantities of the reagents which have been employed in the actual analysis, 1 gram of pure sugar being used in place of the weighed portion of the sample. The quantity of standard acid found to have been neutralised in this control experiment shall be deducted from the total quantity of acid neutralised in the distillation of the sample.
|
|
|
If nitrates are present, the digestion and subsequent distillation shall be carried out as specified in clause (i) of subparagraph (d) of paragraph (5) of Article 9 of these Regulations.
|
|
|
True protein
|
|
|
(c) (i) True protein shall be determined as follows : 0·7 gram of the sample shall be placed in a beaker and 100 millilitres of water shall be added. If the sample is of a substance rich in starch, the mixture shall be heated on a steam bath for 10 minutes ; in any other case, the mixture shall be heated to boiling. There shall be added such a quantity of the cupric hydroxide reagent prepared as described in clause (ii) of this subparagraph, as shall contain about 0·5 gram cupric hydroxide. The mixture shall be stirred thoroughly, filtered when cold, and washed with cold water without removing the precipitate from the filter. The percentage of nitrogen in the precipitate shall be determined as described in subparagraph (c) of paragraph (5) of Article 9 of these Regulations. That percentage multiplied by 6·25, shall be taken to be the percentage of true protein contained in the sample.
|
|
|
If the sample is of a substance rich in alkaline phosphates, 1 or 2 millilitres of 10 per cent. solution of ammonia-free sodium aluminium sulphate shall, before the addition of the cupric hydroxide, be added to the mixture in order to prevent the formation of cupric phosphate and free alkali and the consequent partial solution of the copper-protein precipitate in the alkaline liquid.
|
|
|
(ii) Cupric hydroxide shall be prepared as follows : 100 grams of crystalline copper sulphate shall be dissolved in 5 litres of water. There shall be added 2·5 millilitres of glycerol and then 10 per cent. sodium hydroxide solution until the liquid is slightly alkaline. The mixture shall be filtered and the precipitate rubbed in a mortar with water containing 5 millilitres of glycerol per litre. The precipitate shall be washed by decantation or filtration until the washings are no longer alkaline. The precipitate shall be again rubbed in a mortar with water containing 10 per cent. of glycerol until a uniform gelatinous mass measurable by pipette is produced. The quantity of cupric hydroxide in 5 millilitres shall be determined by diluting to 50 millilitres with water, filtering, washing, igniting, and weighing as cupric oxide.
|
|
|
Determination of fibre.
|
|
|
(6) (a) Determination of fibre shall be effected as specified in the subsequent subparagraphs of this paragraph.
|
|
|
Preparation of Buchner funnel.
|
|
|
(b) The Buchner funnel shall be prepared as follows : The holes shall be covered with a disc of cotton cloth cut from bleached cotton drill conforming to the following specification :
|
|
|
Threads per half inch : Warp, 50 : Weft, 34 : Weight per linear yard, 28 inches wide : 6½ oz.
|
|
|
Boiling water shall be poured into the funnel and allowed to remain until the funnel is hot, whereupon suction shall be applied.
|
|
|
Procedure
|
|
|
(c) 2 or 3 grams, accurately weighed, shall be extracted with petroleum ether (b. pt. 40-60°C.) in an extraction apparatus, the residue dried and transferred to a litre conical flask. The material must not be further ground during extraction. A volume of 200 millilitres of a solution containing 1·25 grams of sulphuric acid per 100 millilitres measured at ordinary temperature and brought to boiling point, shall be added to the flask and heated. The contents of the flask must come to boiling within 1 minute and the boiling throughout must be gentle and continuous for exactly 30 minutes, the original volume being maintained. The flask shall be rotated every few minutes in order to mix the contents and remove particles from the sides. At the end of 30 minutes the flask shall be removed and the contents shall be poured at once into a Buchner funnel prepared as described in the previous subparagraph. After filtration of the bulk of the 200 millilitres
through the Buchner funnel the residue shall be washed with hot water until the washings are free from acid. The residue shall then be washed from the disc of cotton cloth back into the flask with 200 millilitres of a solution of sodium hydroxide, containing 1·25 grams of sodium hydroxide per 100 millilitres free or nearly free from sodium carbonate, measured at ordinary temperature and brought to boiling point. The contents of the flask shall be boiled for exactly 30 minutes, the precautions given for the treatment with acid being observed. At the end of 30 minutes the flask shall be removed and its contents immediately filtered through a disc of cotton cloth, as before. The residue collected on the disc shall be washed with hot water until free from acid and then transferred to a beaker with hot water from a wash bottle. The contents of the beaker shall then be filtered through a prepared Gooch crucible, the beaker and crucible being washed several times with hot water. The residue
in the Gooch crucible shall be washed twice with 95 per cent. alcohol, and 3 times with ether and dried at about 100°C. in an oven until the weight of the crucible and its contents is found to be constant. The contents of the crucible shall then be incinerated at a dull read heat. The weight of the crucible and its contents after incineration shall be subtracted from the weight of the crucible and its contents immediately before incineration and the difference (if any) shall be reported as fibre.
|
|
|
Determination of phosphorus.
|
|
|
(7) Determination of phosphorus shall be effected as follows : a weighed portion of the sample shall be treated as described in clause (iii) of subparagraph (c) of paragraph (6) of Article 9 of these Regulations and the phosphorus in an aliquot part of the solution obtained thereby shall be determined by the method described in clause (iv) of that subparagraph.
|
|
|
Determination of betacarotene.
|
|
|
(8) Determination of betacarotene shall be effected as follows : 1 or 2 grams of the sample, depending on its probable betacarotene content, shall be boiled with 50 millilitres of petroleum ether (b. pt. 80-100°C.) in a large Kjeldahl flask on a steam bath for an hour. The flask and its contents shall then be cooled and the liquid decanted onto a column of bone meal previously wetted with petroleum ether (b. pt. 40-60°C.). Light suction shall be applied to the bone-meal column so that the eluate drops slowly through. The flask and the residue therein shall be repeatedly rinsed with small quantities of petroleum ether (b. pt. 40-60°C.), each rinsing being passed through the column, until a colourless eluate appears. The total eluate obtained shall then be made up to 200 millilitres and the betacarotene content shall be determined colorimetrically by means of an absorptiometer or a spectrophotometer.
|
|
|
Determination of ash.
|
|
|
(9) Determination of ash shall be effected as follows : a weighed quantity of the sample, from 2 to 5 grams, shall be transferred to a suitable dish and incinerated at a temperature not exceeding 500°C. The dish and contents shall be cooled, weighed, and the percentage of ash calculated.
|
|
|
Determination of sand and silicious matter.
|
|
|
(10) Determination of sand and silicious matter shall be effected as follows : the ash obtained by the method described in paragraph (9) of this Article shall be moistened with hydrochloric acid and evaporated to dryness and shall then be repeatedly extracted with hot dilute hydrochloric acid (1 part of concentrated hydrochloric acid to 4 parts of water). The solution shall be filtered and the insoluble matter washed, incinerated and weighed. The quantity obtained shall be taken as sand and silicious matter.
|
|
|
Determination of calcium.
|
|
|
(11) Determination of calcium shall be effected as follows : 0·4 gram of ash obtained by the method described in paragraph (9) of this Article shall be transferred to a 250 millilitre beaker and about 6 millilitres of water and 10 millilitres of concentrated hydrochloric acid shall be added. The beaker and contents shall be heated on a steam bath for a few minutes until decomposition is complete. The solution shall then be diluted to about 50 millilitres and heated almost to boiling. About 100 millilitres of a hot (90°C.) 5 per cent. ammonium oxalate solution, together with a few drops of 0·1 per cent. methyl orange, shall then be added. The calcium oxalate shall be precipitated (at an initial temperature of about 80°C.) by adding successive drops of a solution of ammonia obtained by mixing 1 volume of water with 1 volume of ammonia solution (specific gravity 0·880) until a pH of approximately 4 is seen to be obtained by comparison of the colour of the
solution with the colour of an equal volume of 0·1 molar potassium hydrogen phthallate containing the same quantity of indicator. The solution shall be allowed to stand for 20 minutes and then filtered through a prepared Gooch crucible. The beaker and precipitate shall be well washed with 8 or 10 portions (amounting in all to not more than 100 millilitres) of very cold water. The crucible and its contents shall be transferred to the original beaker, and about 100 millilitres of water and 5 to 6 millilitres of concentrated sulphuric acid shall be added. The solution shall be titrated at about 90°C. with 0·1 N potassium permanganate which shall have been standardised under the same conditions against standard sodium oxalate dried at 110°C. The result shall be calculated in accordance with the formula :
|
|
|
No. of millilitres of 0·1 N K Mn 04 x 1·25==the percentage of CaCO3 present when 0·4 gram sample is used.
|
|
|
Determination of magnesium.
|
|
|
(12) Determination of magnesium shall be effected as follows : an accurately weighed quantity of the sample, equivalent to not more than 2 grams of magnesium, shall be transferred to a large conical flask, to which 30 millilitres of concentrated hydrochloric acid shall be added. The flask shall be rotated so as to facilitate the elimination of carbon dioxide. 70 millilitres of water shall then be added. The solution shall be heated to boiling-point and allowed to stand on the steam-bath for 1 hour. The contents of the flask shall then be filtered, with suction, through a small Buchner funnel and washed with small quantities of hot water. The filtrate shall be cooled and then transferred to a 200 millilitre graduated flask and diluted to volume with water. An aliquot portion (X millilitres) of the filtrate, containing the equivalent of approximately 0·1 gram of magnesium, shall be transferred to a beaker, to which 10 per cent. ammonium carbonate solution shall
be added dropwise until a white precipitate is produced. The precipitate shall be just dissolved by the careful addition of 0·5 N hydrochloric acid solution. 15 per cent. ferric chloride solution shall then be added dropwise until a yellow colour is produced, at which point 2 grams of sodium acetate trihydrate shall be added. The mixture shall be stirred and more ferric chloride added until a brown colour is produced in the solution. The solution shall then be diluted to about 400 millilitres with water, and boiled for 1 minute. The contents of the flask shall then be filtered, with suction, through a Buchner funnel and washed with hot water. The filtrate shall be transferred to a litre beaker and, if necessary, made slightly acid with 0·5 N hydrochloric acid solution. 1 gram of oxalic acid and 2 grams of ammonium chloride shall then be added. The whole shall be heated to boiling-point and made slightly alkaline--to litmus--by the dropwise addition of dilute ammonia
solution. The precipitate shall be allowed to settle for no more than 15 minutes, then filtered, with suction, through a Buchner funnel and washed with hot water. The filtrate shall be transferred to a large beaker and 10 millilitres of 20 per cent. diammonium hydrogen phosphate solution added, following which the whole shall be boiled. (If a precipitate is produced at this stage it shall be dissolved by the addition of the minimum quantity of concentrated hydrochloric acid). 30 millilitres of strong solution of ammonia shall then be added. The beaker shall be allowed to stand on the steam-bath for at least 4 hours. Its contents shall then be filtered through a filter paper and washed with 2 per cent. ammonia solution until free from chloride. The filter paper shall be transferred to a tared platinum crucible, moistened with a few drops of ammonium nitrate solution, dried, and finally ignited in a muffle furnace to constant weight. The content of magnesium present in the sample shall
be calculated in the following manner :--
|
|
|
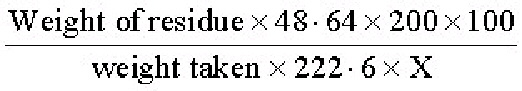
|
|
|
Determination of salt (sodium chloride).
|
|
|
(13) Determination of salt (sodium chloride) shall be effected as follows : 5 grams of the sample shall be mixed with 1 gram of pure sodium carbonate and thoroughly wetted with a little water. The mixture shall be dried and heated to a temperature not exceeding 500°C. in order to destroy organic matter. The residue shall be extracted with water, the volume made up to 250 millilitres and the solution filtered. The chlorine shall be determined, by titration with silver nitrate, in an aliquot portion of the filtrate and the percentage of sodium chloride calculated.
|
|
|
Determination of manganese (acid soluble).
|
|
|
(14) (a) Determination of manganese (acid soluble) shall be effected as specified in the subsequent subparagraphs of this paragraph.
|
|
|
(b) A reagent shall be prepared as follows : 1·4383 grams of potassium permanganate shall be dissolved by boiling with water, then diluted to 1 litre and allowed to stand for several days. The solution shall then be filtered through an asbestos pad on a Gooch crucible and standardised with sodium oxalate. The solution so obtained shall contain at least 500 parts per million of manganese. An aliquot part of the solution containing 20 milligrams of manganese shall be transferred to a beaker. 100 millilitres of water, 15 millilitres of orthophosphoric acid and 0·3 gram of potassium periodate shall then be added. The solution shall be brought to boiling point, cooled and then diluted to 1 litre. The solution shall be protected from light. The solution (containing 20 parts per million of manganese) shall be diluted with water which has been boiled with 0·3 gram of potassium periodate per litre, to make convenient working standards of known concentrations
approximately the same as those to be compared.
|
|
|
Procedure
|
|
|
(c) The procedure shall be as follows : a weighed quantity of the sample containing from 5 to 15 grams shall be incinerated at dull red heat in a porcelain dish and subsequently cooled. The ash so obtained shall be transferred to a beaker with 20 to 30 millilitres of water ; 5 millilitres of concentrated sulphuric acid and 5 millilitres of concentrated nitric acid shall then be added and the mixture evaporated to white fumes. Further quantities of concentrated nitric acid may be added until the carbon is destroyed. The mixture shall then be cooled slightly and transferred to a 100 millilitre volumetric flask. 50 millilitres of dilute orthophosphoric acid (8 + 92) shall then be added. The mixture shall be cooled and diluted to volume with water, mixed and either filtered or allowed to stand until clear. 50 millilitres of clear solution shall then be pipetted into a 100 millilitre flask, followed by 30 millilitres of water. The solution so obtained shall
be heated nearly to boiling point with stirring. 0·3 gram of potassium periodate shall be added for each 15 milligrams of manganese present. The solution shall be maintained at 90-100°C. for 30 to 60 minutes or until colour development is complete. The solution shall next be cooled and diluted to 100 millilitres ; and shall then be compared with standard potassium permanganate solution (prepared as described in subparagraph (b) of this paragraph) using a colorimeter or spectrophotometer at 530 mµ. The content of manganese present in the sample shall then be calculated.
|
|
|
Determination of cobalt.
|
|
|
(15) (a) Determination of cobalt shall be effected as specified in the subsequent subparagraphs of this paragraph.
|
|
|
Reagents.
|
|
|
(b) Reagents shall be prepared as follows :
|
|
|
(i) Cobalt sulphate solution : 0·2385 gram of cobalt sulphate shall be dissolved in water and diluted to 1 litre. (1 millilitre of this solution is equal to 0·05 milligram of cobalt). The solution shall be diluted to a suitable concentration to prepare standard curve.
|
|
|
(ii) Nitroso--R salt solution : 1 gram of Nitroso--R salt shall be dissolved in water and diluted to 500 millilitres.
|
|
|
(iii) Spekker acid : 150 millilitres of 85 per cent. orthophosphoric acid shall be mixed with 150 millilitres of concentrated sulphuric acid and the mixture diluted with water to 1 litre.
|
|
|
(iv) Sodium acetate solution : 500 grams of crystalline sodium acetate shall be dissolved in water and the solution made up to 1 litre.
|
|
|
Standard curve
|
|
|
(c) A standard curve shall be prepared as follows : to 1, 2, 3 etc. millilitres of reagent (i) in 100 millilitre volumetric flasks shall be added 2 millilitres of reagent (iii), 10 millilitres of reagent (ii) and 10 millilitres of reagent (iv), A " blank " shall be prepared by using 2 millilitres of reagent (iii) and 10 millilitres of reagent (iv), omitting reagent (ii). The standards and " blank " shall be brought to the boil on a hot plate. 5 millilitres of concentrated nitric acid shall be added and the solutions boiled for a period between 1 and 2 minutes. The solutions (standards and blank) shall be cooled and diluted with water to 100 millilitres. A standard curve shall then be plotted from these solutions using a colorimeter or spectrophotometer.
|
|
|
Procedure.
|
|
|
(d) The procedure shall be as follows : 2 grams of the sample shall be weighed and incinerated for 2 hours at 600°C. The ash shall be transferred to a 200 millilitre volumetric flask with 20 millilitres of hydrochloric acid and 50 millilitres of water. The mixture shall be boiled for 5 minutes, cooled and diluted to volume with water. The precipitate shall be allowed to settle and a suitable aliquot of the clear liquid shall be pipetted into a small flask. (In the case of samples containing 0·01 to 0·2 per cent. cobalt, an aliquot corresponding to 0·25 gram of the sample shall be used. In the case of other samples the aliquot shall be so chosen that it contains not more than 0·5 milligram of cobalt).
|
|
|
A brisk current of hydrogen sulphide shall then be passed through the solution for 10 minutes. The solution shall next be filtered into a 100 millilitre volumetric flask through Whatman No. 40 filter paper and then washed with about 50 millilitres of 1 per cent. sulphuric acid, which has been saturated with hydrogen sulphide. 2 small glass beads shall be added and the hydrogen sulphide shall then be boiled off. Each flask shall be given individual attention and shaken often. 5 millilitres of concentrated citric acid shall then be added and the solution boiled until nitrous fumes no longer appear. The solution shall be removed from the hot plate at first indication of bumping and cooled. 2 drops of phenolphthalein shall be added and the solution brought to the first faint pink with about a 30 per cent. sodium hydroxide solution. 2 millilitres of reagent (iii) shall immediately be added, followed by 10 millilitres of reagent (ii) and 10 millilitres of reagent
(iv). The mixture shall then be brought to boil vigorously. 5 millilitres of concentrated nitric acid shall then be carefully added, and the mixture boiled for not less than 1 but not more than 2 minutes. The solution shall be cooled and diluted to volume with water. Its colour shall be compared with that of standard cobalt solution, in a colorimeter (using green or No. 54 filter) or in spectrophotometer at 540 mµ. The colour shall be read within 2 hours and the content of cobalt present in the sample shall then be calculated from the standard curve.
|
|
|
Determination of Copper.
|
|
|
(16) (a) Determination of copper shall be effected as specified in the subsequent subparagraphs of this paragraph.
|
|
|
Preparation of standard curve.
|
|
|
(b) A standard curve shall be prepared as follows : 1·9645 grams of crystalline copper sulphate shall be dissolved in water and diluted to 500 millilitres. (1 millilitre of this solution is equal to 1 milligram of copper). From 1 to 10 millilitres of this solution shall be used to prepare a set of standards in 100 millilitre Pyrex glass-stoppered volumetric flasks. 4 millilitres of concentrated hydrochloric acid shall be added and the solution diluted to 50 millilitres. 5 millilitres of tetraethylenepentamine shall then be added ; the solution so obtained shall be diluted to mark with water, stoppered and subsequently mixed. A " blank " shall be prepared by using all reagents except copper. The standards and " blank " shall be filtered before reading colour, as indicated in the determination referred to in the next subparagraph. A standard curve shall then be plotted from these solutions, using a colorimeter or spectrophotometer.
|
|
|
Procedure.
|
|
|
(c) The procedure shall be as follows : 8 grams of the sample shall be weighed and incinerated for 2 hours at 600°C. The ash shall be transferred to a 200 millilitre volumetric flask with 20 millilitres of hydrochloric acid and 50 millilitres of water. The mixture shall be boiled for 5 minutes, cooled and diluted to volume with water. The precipitate shall be allowed to settle. A 50 millilitre aliquot of the clear liquid shall be pipetted into a 100 millilitre Pyrex glass-stoppered volumetric flask. 5 millilitres of tetraethylenepentamine shall be added, the solution diluted to volume with water, stoppered and mixed thoroughly. The solution shall then be filtered and the colour compared with the standards within 30 minutes in a colorimeter (red or No. 66 filter) or shall be read in a spectrophotometer at 620 mµ. The content of copper present in the sample shall then be calculated from the standard curve.
|
|
|
Determination of Iodine.
|
|
|
(17) Determination of iodine shall be effected as follows : a weighed portion of the sample containing 3 to 4 milligrams of iodine shall be placed in a 200-300 millilitre nickel dish. There shall be added about 5 grams of anhydrous sodium carbonate, 5 millilitres of sodium hydroxide solution (1 + 1) and 10 millilitres alcohol, care being taken to ensure that the entire sample is moist. The dish and its contents shall be dried at 100°C. for about 30 minutes and then heated in a muffle furnace to 500°C. the temperature being maintained at 500°C. for 15 minutes. The dish and contents shall then be cooled. 25 millilitres of water shall be added and the dish then covered with a watch glass, the contents being boiled gently for 10 minutes. The contents of the dish shall then be transferred on to filter paper 18 centimetres in diameter. The dish and paper shall be washed with boiling water into a 600 millilitre beaker until the filtrate is approximately 300
millilitres. The filtrate shall be neutralised to methyl orange with 85 per cent. orthophosphoric acid adding 1 millilitre of acid in excess. Excess bromine water shall be added and the solution boiled until colourless and for a further five minutes thereafter. A few crystals of salicylic acid shall then be added and the solution cooled to about 20°C. 1 millilitre of 85 per cent. orthophosphoric acid and about 0·5 gram of potassium iodide shall be added and the solution titrated with 0·005 N sodium thiosulphate. The content of iodine present in the sample shall then be calculated.
|
|
|
Determination of Iron.
|
|
|
(18) (a) Determination of iron shall be effected as specified in the subsequent subparagraphs of this paragraph.
|
|
|
Preparation of standard curve.
|
|
|
(b) A standard curve shall be prepared as follows : a solution containing 50 parts per million solution of iron shall be prepared and the following quantities transferred to colorimeter tubes marked at 10 millilitres--0·4, 0·8, 1·2, 1·6 and 2·0 millilitres, giving 20, 40, 60, 80 and 100 milligrams of iron respectively. To each tube shall then be added 1 millilitre of 20 per cent. citric acid solution and sufficient distilled water to bring the volume to approximately 5 millilitres. A " blank " shall be prepared by adding to another tube 1 millilitre of 20 per cent. citric acid solution and 4 millilitres of distilled water. To each tube shall be added 3 drops of thioglycollic acid followed, after the contents have been thoroughly mixed, by such quantity (about 4 millilitres) of 10 per cent. ammonia solution as will suffice to produce a stable reddish colour. The contents of each tube shall then be diluted to volume with water and mixed thoroughly. A
standard curve shall then be plotted from these solutions using a colorimeter or spectrophotometer.
|
|
|
Procedure.
|
|
|
(c) The procedure shall be as follows : a sample containing about 1 milligram of iron shall be transferred to a 100 millilitre conical flask. 4 grams of citric acid and 80 millilitres of water shall then be added. The contents shall be well mixed and then allowed to stand for about 1 hour. The mixture shall then be diluted to volume and filtered. A 5 millilitre aliquot of the solution so obtained shall be transferred to a marked colorimeter tube, to which 3 drops of thioglycollic acid shall be added, followed by 10 per cent. ammonia solution dropwise until a stable reddish colour develops (care being taken to avoid adding excess ammonia). This solution shall be diluted to 10 millilitres with water and the colour compared with the standards in a colorimeter or spectrophotometer. The content of iron present in the sample shall then be calculated from the standard curve.
|
|
|
Form of certificate of result of analysis. |
|
|
11. For the purposes of sections 3 and 8 of the Act, the prescribed form of the result of the analysis shall be such one of the forms set out in the Third Schedule to these Regulations as may be applicable to the case.
|
|
|
Prohibition of sale and manufacture for sale of certain compound feeding stuffs |
|
|
12. A person shall not sell or manufacture for sale any compound feeding stuff the ingredients of which include any one or more of the following articles :
|
|
|
(a) cocoa shells whether alone or mixed with any other article and whether whole or ground,
|
|
|
(b) corozo nut meal,
|
|
|
(c) grass seed cleanings,
|
|
|
(d) hoof meal or horn meal or any other substance produced by the grinding of hoofs or horns,
|
|
|
(e) husks derived from the milling or dehulling of oats, hulls so derived, or shudes so derived, whether such husks, hulls or shudes are alone or are mixed with any other article and whether they are whole or ground,
|
|
|
(f) a by-product of oat-milling which contains, if it is unground, more than 20 per cent. of fibre or, if it is ground, more than 16 per cent. of fibre.
|
|
|
Restriction of manufacture for sale of fertilisers, compound feeding stuffs and mineral mixtures. |
|
|
13.--(1) A person shall not manufacture for sale--
|
|
|
(a) any fertiliser,
|
|
|
(b) any compound feeding stuff other than any mixture which consists solely of--
|
|
|
(i) any two or more of the following articles : ground barley, flaked barley, crushed barley, ground wheat, flaked wheat, crushed wheat, ground maize, flaked maize, crushed maize, ground milo maize, flaked milo maize and crushed milo maize,
|
|
|
(ii) two or more by-products of oat-milling none of which is husks derived from the milling or dehulling of oats, hulls so derived, or shudes so derived, or
|
|
|
(iii) molasses and sugar beet pulp, or
|
|
|
(c) any mineral mixture,
|
|
|
save under and in accordance with a licence granted to him by the Minister under these Regulations.
|
|
|
(2) A licence granted by the Minister under
|
|
|
(a) the emergency Powers (Manufacture and Sale of Fertilisers) Order, 1944 (S. R. & O. No. 49 of 1944), or
|
|
|
(b) Article 3 of the Emergency Powers (Feeding Stuffs) Order, 1944 (S. R. & O. No. 196 of 1944), or
|
|
|
(c) the Emergency Powers (Manufacture and Sale of Mineral Mixtures) Order, 1944 (S. R. & O. No. 50 of 1944),
|
|
|
in force immediately before the commencement of these Regulations shall, on and after such commencement, be deemed to be a licence under these Regulations.
|
|
|
Prohibition of sale of certain articles as feeding stuffs. |
|
|
14. A person shall not sell as a feeding stuff--
|
|
|
(a) any cocoa shells, whether whole or ground,
|
|
|
(b) any husks derived from the milling or dehulling of oats, hulls so derived, or shudes so derived, whether such husks, hulls, or shudes are alone or are mixed with any other article and whether they are whole or ground,
|
|
|
(c) any article which--
|
|
|
(i) has been produced by grinding any by-product of oat-milling other than husks, hulls, or shudes, or
|
|
|
(ii) is a mixture, whether whole or ground, of two or more such by-products and contains more than 16 per cent. of fibre, or
|
|
|
(d) any article which purports to consist wholly or mainly of oatmeal and which contains more than 5 per cent. of fibre.
|
|
|
Restriction of sale of grass meal. |
|
|
15.--(1) A person shall not sell grass meal under the description of High Grade or Grade I unless it contains at least 16 per cent. protein and 220 parts per million of carotene.
|
|
|
(2) A person shall not sell grass meal under the description of Standard Grade or Grade II unless it contains at least 14 per cent. protein and 150 parts per million of carotene.
|
|
|
(3) A person shall not sell as a feeding stuff any grass meal unless it contains at least 14 per cent. protein.
|
|
|
Restriction of sale of meat and bone meal. |
|
|
16. A person shall not sell any feeding meat and bone meal containing less than 40 per cent. of protein.
|
|
|
Exception for sales for export. |
|
|
17. Sales for export shall be excepted from the application of Articles 14, 15 and 16 of these Regulations.
|
|
|
Prohibition of bringing of certain articles into certain premises. |
|
|
18. A person shall not bring into any premises where any feeding stuff, compound feeding stuff or mineral mixture intended for sale is manufactured, packed, stored or sold, any husks derived from the milling or dehulling of oats, hulls so derived, or shudes so derived, whether such husks, hulls, or shudes are alone or are mixed with any other article and whether they are whole or ground.
|
|
|
Article 3 (1).
|
|
|
FIRST SCHEDULE.
|
|
|
A mixture consisting solely of any two or more of the following articles : ground barley, flaked barley, crushed barley, ground wheat, flaked wheat, crushed wheat, ground maize, flaked maize, crushed maize, ground milo maize, flaked milo maize and crushed milo maize.
|
|
|
Antibiotic feed supplements.
|
|
|
Barley meal.
|
|
|
Bean meal.
|
|
|
Beet pulp (whether wet, dried or pressed).
|
|
|
Brewery grains, distillery grains or grains part of which consists of brewery grains and the remainder of which consists of distillery grains.
|
|
|
Dried molassed beet pulp.
|
|
|
Flaked barley.
|
|
|
Flaked maize.
|
|
|
Flaked milo maize.
|
|
|
Flaked oatmeal.
|
|
|
Flaked wheat.
|
|
|
Ground oats, crushed oats or rolled oats.
|
|
|
Ground wheat.
|
|
|
Maize meal (Indian meal).
|
|
|
Milo maize meal.
|
|
|
Molasses or treacle.
|
|
|
Oatmeal.
|
|
|
Pea meal.
|
|
|
Vitamin supplements containing synthetic vitamins.
|
|
|
Wheat offals.
|
|
|
Article 3 (2) (e).
|
|
|
SECOND SCHEDULE.
|
|
|
|
|
|
|
Article
|
Particulars to be contained in Statement
|
|
Blood meal
|
Amount of oil ; minimum amount of crude protein.
|
|
Bone flour or bone meal
|
Minimum amounts of phosphorus and calcium, respectively.
|
|
Coconut or copra cake or meal
|
Amount of oil ; minimum amount of crude protein.
|
|
Cotton cake or meal or cotton seed meal
|
Amount of oil ; minimum amount of crude protein; whether decorticated or undecorticated.
|
|
Dried yeast
|
Minimum amount of crude protein.
|
|
Earth nut or ground nut cake or meal
|
Amount of oil ; minimum amount of crude protein ; whether decorticated or undecorticated.
|
|
Feeding meat and bone meal, or any other product of meat (including whale meat) and bone for feeding purposes
|
Amount of oil ; minimum amounts of crude protein, phosphorus and calcium, respectively.
|
|
Feeding meat meal, or any other product of meat (including whale meat) for feeding purposes
|
Amount of oil ; minimum amounts of crude protein, phosphorus and calcium respectively.
|
|
Fish meal--including any product (other than shark meal) obtained by drying and grinding or otherwise treating fish or fish waste
|
Amount of oil ; minimum amounts of crude protein, phosphorus and calcium, respectively.
|
|
|
|
|
|
|
|
Article
|
Particulars to be contained in Statement
|
|
Grass meal, lucerne meal, or clover meal--in any form
|
Minimum amounts of crude protein and betacarotene, respectively.
|
|
Linseed cake or meal
|
Amount of oil ; minimum amount of crude protein.
|
|
Locust bean meal
|
Amount of oil ; minimum amount of crude protein.
|
|
Malt culms
|
Minimum amount of crude protein ; maximum amount of fibre.
|
|
Oat dust or other by-product of oat milling
|
Minimum amount of crude protein ; maximum amount of fibre.
|
|
Palm kernel cake or meal
|
Amount of oil ; minimum amount of crude protein.
|
|
Rape cake or meal
|
Amount of oil ; minimum amount of crude protein.
|
|
Sesame cake or meal
|
Amount of oil ; minimum amount of crude protein.
|
|
Shark meal
|
Amount of oil ; minimum amount of true protein.
|
|
Soya bean cake or meal
|
Amount of oil ; minimum amount of crude protein.
|
|
Sunflower cake or meal
|
Amount of oil ; minimum amount of crude protein; whether decorticated or undecorticated.
|
|
|
|
Article 11.
|
|
|
THIRD SCHEDULE.
|
|
|
|
|
|
|
|
|
|
FORM F.F.1 (a).
|
Ref. No................
|
|
|
|
|
|
|
CERTIFICATE OF RESULT OF ANALYSIS OF A LIMING MATERIAL.
|
|
|
Sample of............................................................ ...marked............................................................ ....................
|
|
|
received by the State Chemist on............................................................ .......................................................
|
|
|
from............................................................ ............................................................ ........................................
|
|
|
(taken at the premises of............................................................ ............................................................ .....).
|
|
|
This is to certify that the above-mentioned sample, which was duly fastened and sealed, has been analysed by...................................... under the direction of the State Chemist or the Assistant State Chemist and that the result of the analysis is as follows :--
|
|
|
|
|
|
|
per cent.
|
|
Neutralising value, expressed in terms of calcium carbonate
|
.....................
|
|
Fineness:
|
|
|
(1) Amount found to pass through a prescribed 1/8 inch sieve
|
.....................
|
|
(2) Amount found to pass through a prescribed No. 100 sieve
|
.....................
|
|
|
|
*On comparison of the result of the analysis with the particulars accompanying the sample it appears that the particulars furnished--
|
|
|
*are correct, subject to the prescribed limits of error.
|
|
|
*are not correct, subject to the prescribed limits of error, in the following respects :--
|
|
|
This certificate is given under section *3/8* of the Fertilisers, Feeding Stuffs and Mineral Mixtures Act, 1955.
|
|
|
|
|
|
|
|
|
|
Date....................
|
Signed...................
|
|
|
|
|
|
|
State Chemist.
|
|
|
*Delete if inapplicable.
|
|
|
|
|
|
|
|
|
|
Form F. F. 1 (b).
|
Ref. No...............
|
|
|
|
|
|
|
CERTIFICATE OF RESULT OF ANALYSIS OF A FERTILISER OTHER THAN A LIMING MATERIAL.
|
|
|
Sample of............................................................ .. marked............................................................ .............
|
|
|
received by the State Chemist on............................................................ ................................................
|
|
|
from............................................................ ............................................................ .......................................
|
|
|
(taken at the premises of............................................................ ............................................................ ..).
|
|
|
This is to certify that the above-mentioned sample, which was duly fastened and sealed, has been analysed by...........................under the direction of the State Chemist or the Assistant State Chemist and that the result of the analysis is as follows :
|
|
|
|
|
|
|
|
|
Per cent.
|
|
Nitrogen
|
|
|
.......................................
|
|
Phosphorus
|
 |
Water Soluble
|
......................................
|
|
Citric Acid Soluble
|
.......................................
|
|
Citrate Soluble
|
.......................................
|
|
*Water/*Citric Acid/*Citrate insoluble.
|
.......................................
|
|
Potassium
|
|
|
......................................
|
|
*Amount found to pass through a prescribed No. 100 sieve
|
.......................................
|
|
|
|
*On comparison of the results of the analysis with the particulars accompanying the sample it appears that the particulars furnished--
|
|
|
*are correct, subject to the prescribed limits of error.
|
|
|
*are not correct, subject to the prescribed limits of error, in the following respects :--
|
|
|
This certificate is given under section *3/*8 of the Fertilisers, Feeding Stuffs and Mineral Mixtures Act, 1955.
|
|
|
Date....................................................... Signed....................................
|
|
|
State Chemist.
|
|
|
|
|
|
|
Form F.F.2.
|
Ref. No................
|
|
|
|
|
|
|
CERTIFICATE OF RESULT OF ANALYSIS OF A FEEDING STUFF OR A COMPOUND FEEDING STUFF (OTHER THAN A MINERAL MIXTURE).
|
|
|
Sample of............................................................ ............. marked............................................................ ......
|
|
|
received by the State Chemist on............................................................ .......................................................
|
|
|
from............................................................ ............................................................ .........................................
|
|
|
(taken at the premises of............................................................ ............................................................ .......)..
|
|
|
This is to certify that the above-mentioned sample, which was duly fastened and sealed, has been analysed by............................ under the direction of the State Chemist or the Assistant State Chemist and that the result of the analysis is as follows :--
|
|
|
|
|
|
|
Per cent.
|
|
Oil
|
...................................
|
|
Protein (*crude/*true)
|
...................................
|
|
Fibre
|
...................................
|
|
|
|
*On comparison of the result of the analysis with the particulars accompanying the sample it appears that the particulars furnished--
|
|
|
*are correct, subject to the prescribed limits of error.
|
|
|
*are not correct, subject to the prescribed limits of error, in the following respects :--
|
|
|
*Delete if inapplicable.
|
|
|
This certificate is given under section *3/*8 of the Fertilisers, Feeding Stuffs and Mineral Mixtures Act, 1955.
|
|
|
Date............................................................ .......... Signed..............................................
|
|
|
State Chemist.
|
|
|
|
|
|
|
|
|
|
Form F.F.3.
|
Ref. No...................
|
|
|
|
|
|
|
CERTIFICATE OF RESULT OF ANALYSIS OF A MINERAL MIXTURE.
|
|
|
Sample of............................................................ ....... marked............................................................ ...............
|
|
|
received by the State Chemist on............................................................ .......................................................
|
|
|
from............................................................ ............................................................ ..........................................
|
|
|
(taken at the premises of............................................................ ............................................................ ....)..
|
|
|
This is to certify that the above-mentioned sample, which was duly fastened and sealed, has been analysed by.................... under the direction of the State Chemist or the Assistant State Chemist and that the result of the analysis is as follows :--
|
|
|
|
|
|
|
Per cent.
|
|
Calcium
|
....................................
|
|
Phosphorus
|
....................................
|
|
Salt (sodium chloride)
|
....................................
|
|
Magnesium
|
....................................
|
|
Manganese (acid soluble)
|
....................................
|
|
Cobalt
|
....................................
|
|
Copper
|
....................................
|
|
Iodine
|
....................................
|
|
Iron
|
....................................
|
|
|
|
*On comparison of the result of the analysis with the particulars accompanying the sample it appears that the particulars furnished--
|
|
|
*are correct, subject to the prescribed limits of error.
|
|
|
*are not correct, subject to the prescribed limits of error, in the following respects :--
|
|
|
This certificate is given under section *3/8* of the Fertilisers, Feeding Stuffs and Mineral Mixtures Act, 1955.
|
|
|
Date............................................................ ........ Signed..............................................
|
|
|
State Chemist.
|
|
|
GIVEN under my Official Seal, this 20th day of December, 1957.
|
|
|
(Signed) PATRICK SMITH
|
|
|
Minister for Agriculture.
|
|
|
EXPLANATORY NOTE.
|
|
|
The Regulations control the sale and manufacture for sale of fertilisers, feeding stuffs and mineral mixtures and provide for the sampling and analysis of such articles.
|
|
|
*Delete if inapplicable.
|
|
|
*Delete if inapplicable.
|
|
|
|